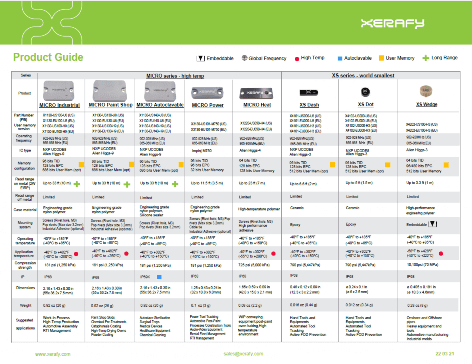
RFID for Healthcare
RFID helps hospitals, device manufacturers, and distributors tracking and digitizing critical inventory.
Equipment
Systems
Trays
Supplies
Equipment
Asset tracking in hospitals has a direct impact on patient care. RFID can be used to track medical equipment like hospital beds and non-stationary machines within an institution.
An RFID hospital equipment tracking system provides the last-known or even real-time location of a piece of equipment, using Real-Time Location System (RTLS) built on passive UHF RFID to provide cost-effective healthcare asset management solutions.
Contactless tracking and automated operation are two additional advantages of RFID for healthcare, especially when compared to barcode-based solutions.
Automated asset tracking saves hospitals time and helps them avoid unnecessary purchases to replace hard-to-locate equipment. With inventories of hundreds of pieces of equipment across large facilities nurses waste precious time searching for a wheelchair or IV pump, or surgeries may be delayed because a critical piece of equipment can’t be found.
Systems
Medical device manufacturers and distributors offer RFID-enabled loaner system services for procedures relying on loaned instrumentation and consigned inventories of implants and devices.
Surgical sets and implants are kept in metal and plastic trays that are designed to handle the devices during transport and protect them during sterilization. RFID-enabled containers are now available with embedded tracking capabilities to fully digitize their inventory management in the trunk, in the field, or at the institution.
With manufacturers required to provide UDI traceability for their inventory, RFID technology has emerged as a critical component of the Patient Safety agenda pushed by a number of healthcare regulators around the world, including the FDA.
What began as an insurmountable challenge is now viewed as a once-in-a-lifetime opportunity, with medical device manufacturers leveraging the technology to enable added-value services for patients and healthcare providers such as fully automated reconciliation, streamlined procurement, improved recall programs, and anonymous usage data.
Trays
Hospitals use RFID systems to accurately track and control their inventory of surgical trays, containers, and endoscopes, making them safer for patients and more efficient to run.
They help the perioperative nurses in the OR with everything from case scheduling to surgical counts. They also assist SPD personnel with reprocessing and sterilization procedures.
The systems are designed and deployed in hospitals by specialist Xerafy partners using the company’s autoclavable RFID tags.
Supplies
Hospitals operate some extraordinarily complex and critical supply chains, with medical RFID enabling automation and new levels of operational efficiency.
Patient safety improves when UDI-compliant traceability is implemented, products are authenticated and substance distribution is effectively controlled.
On the other hand, hospitals increase their operational efficiency by automating stock replenishment, tracking deliveries, optimizing just-in-time inventories with real-life and real-time data analytics, and closely supervising consigned inventories and controlled substances.
Benefits of RFID For Hospitals
Real-Time Accuracy
Lost medical equipment is costly and RFID provides full tracking accuracy based on last-known or real-time location.
Productivity
RFID tracking saves hospitals time by reducing the effort required to manually track equipment and helping quickly locate items, preventing delays in surgeries or patient care.
Automation
Unlike barcode-based solutions, RFID offers automated and contactless tracking, at item-level and in bulk, across large facilities.
Asset Management
RFID helps hospitals avoid unnecessary purchases to replace hard-to-locate equipment and optimize hospital asset management.
Analytics
Assess hospital inventory and usage, allowing to identify any gaps or shortages in medical supplies and make informed decisions about purchasing.
What are the challenges of RFID technology in hospitals?
FDA
How can we avoid adding risk at the point of care? When it comes to the question of RFID technology and Patient Safety, the FDA has adopted a neutral stance: "The FDA is not aware of any adverse events associated with RFID."
Sterile Processing
Sterile processing is not technology-friendly, be it for the sterilization processes such as Autoclave, EtO, E-Beam, Gamma Radiation, or the numerous chemical cleaners in use.
UDI
Compliance with FDA UDI (Unique Device Identification) requirements has become critical for medical device manufacturers
Wireless
Healthcare institutions present a high density of metallic surfaces and assets, which can be challenging for radio frequency identification and other wireless technologies
Inobtrusive
Technology is expected to be inobtrusive, with form factors and tagging systems adapted to every lifecycle and workflow
Can RFID survive autoclave and sterilization?
Yes, Xerafy offers several specialized tagging options that are autoclavable, as well as solutions that can be embedded in metal, with additional customization services available.
Xerafy created the market of RFID for Healthcare, with innovations such as the smallest RFID tags, Autoclavable, High Temperatures, Chemicals, Shocks.
How can an institution get started with RFID tracking?
By deploying the right RFID tracking system, a hospital can hardwire safety and traceability into their processes, enabling them to automatically track, monitor, and safeguard instruments, equipment, supplies, and staff.
PATIENT SAFETY
#1 priority for everyone from the institutions to the regulatory agencies, with a stringent evaluation of risks and benefits that applies to any new system.
UDI Traceability
The worldwide agenda for traceability gives momentum to device manufacturers looking to leverage technology enablement.
Automation
Tracking and monitoring automation helps hospitals incorporate safety and UDI traceability into their operations.
Sterilization
Reprocessing procedures are based on manufacturers' IFUs and a facility’s own best practices for cleaning, chemical treatments, and sterilization parameters by autoclave, EtO, E-Beam, or radiation.
Devices
Tracking technology provides insight into managing field inventory, trunk stock, and consignment programs.
Equipment
Surgical equipment, beds, and stretches are easier and less contentious to track than patients and staff. There are tagging solutions available for any asset, workflow, and lifecycle.
What is the added value of RFID for UDI?
How does RFID technology improve operational efficiency in hospitals?
How does RFID technology prevent unauthorized personnel from accessing hospital premises?
How does RFID technology help monitor patients and improve their safety?
Get In Touch
If you have any questions or need help, feel free to contact with our team.